
Grade 8 | Lesson 16
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com

Grade 8 | Lesson 16
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com
Science
Lesson Overview
The Properties of Metals, Non-Metals and Synthetics
• Properties of Metal Elements
• Properties of Non-Metal Elements
• Synthetic Elements
![]()
The Properties of Metals and Non-Metals
Properties of Metal Elements
Gold, copper, silver, tin, iron, and aluminum are typical metals. What do these and other metals have in common? Most metals are hard, shiny solids. Metals are also good conductors of both heat and electricity. These properties make metals suitable for uses ranging from kitchen pots and pans to wires for electric appliances. Because metals reflect light well, they are also used in mirrors. Metals are malleable, which means they can be hammered or rolled into sheets. Metals are also ductile, which means they can be drawn into wires.
The atoms of metals generally have from one to three electrons in their outer energy levels. Metals tend to give up electrons easily. The atoms of the metals tend to lose electrons to the atoms of nonmetals, and form ionic bonds. The second type of bond you studied is the covalent bond, which generally forms between atoms on nonmetals. A third type of bonding, neither ionic nor covalent, occurs among the atoms in a metal. In metallic bonding, positively charged metallic ions are surrounded by a "sea of electrons." Outer-level electrons are not held tightly to their particular nucleus. Rather, the electrons move freely among many positively charged ions.
The elements in Group 1 of the periodic table are the alkali metals. Like other metals, Group 1 metals are shiny, malleable, and ductile. They are good conductors of both heat and electricity. The alkali metals are highly reactive metals. Each atom of an alkali metal has one electron in its outer energy level. This electron is given up when an alkali metal combines with another atom. The result is a positively charged ion in a compound such as sodium chloride, NaCl, or potassium bromide, KBr. Alkali metals and their compounds have many uses. You and other living things need potassium and sodium compounds - such as table salt, NaCl - to stay healthy. Doctors use lithium compounds to treat bipolar disorder. The operation of some photocells depends upon rubidium or cesium compounds.
Francium, the last element in Group 1, is extremely rare and also radioactive. The nucleus of a radioactive element breaks down and gives off particles and energy.
The alkaline earth metals make up Group 2 of the periodic table. Like the alkali metals, these metals are shiny, malleable, ductile, and so reactive that they are not found free in nature. Each atom of an alkaline earth metal has two electrons in its outer energy level. These electrons are given up when an alkaline earth metal combines with another element. The result is a positively charged ion in a compound such as calcium fluoride, CaF₂.
An iron nail, a copper wire, and a silver dime are examples of objects made from transition elements. The transition elements are those elements in Groups 3 through 12 of the periodic table. Typical transition elements are metals and have one or two electrons in the outer energy level. These metals are less active than those in Groups 1 and 2.
Gems contain brightly colored compounds of transition elements. Brilliant cadmium yellow and cobalt blue paint pigments are made from compounds of transition elements. But cadmium and cobalt paints are so toxic that their use is now limited.
When we examine pictures of metals and their compounds we are really seeing only half the story. Earth's crust contains many compounds and a few examples of uncombined metals such as gold and copper. Metals must be dug, or mined, from Earth's hardened outer layer. Iron is second only to aluminum among the metals in abundance in Earth's crust. As the main component of steel, iron is the most widely used of all metals. Some steels also contain cobalt. Nickel is added to other metals to give them strength. Nickel is also often used to give a shiny, protective coating to other metals.
Properties of Non-Metal Elements
This figure to the right shows that you're mostly made of oxygen, carbon, hydrogen, and nitrogen. Calcium, a metal, and other elements make up the remaining four percent of your body's weight. Phosphorus, sulfur, and chlorine are among other elements found in your body. These elements are classified as nonmetals.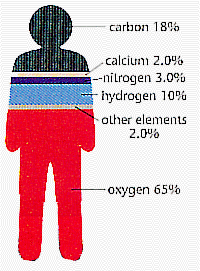
Research It!
How many elements are nonmetals? Most nonmetals are gases at room temperature. Several nonmetals are solids, and one nonmetal is a liquid.
In contrast to metals, solid nonmetals are dull. Because they are brittle and powdery, they are neither malleable nor ductile. The electrons in most nonmetals are tightly attracted and are restricted to one atom. So, as a group, nonmetals are poor conductors of heat and electricity. Let's go through some specifics about different non-metals and groups of them.
Hydrogen
Did you know that approximately 90 percent of all the atoms in the universe are hydrogen? Most hydrogen on Earth is found in the compound water. When water is broken down into its elements, hydrogen forms as a gas made up of diatomic molecules. A diatomic molecule consists of two atoms of the same element. Thus, the formula for hydrogen gas is H₂.
The Halogens
Because an atom of a halogen has seven electrons in its outer energy level, only one electron is needed to complete this energy level. If a halogen gains an electron from a metal, an ionic compound, called a salt, is formed. The word halogen means "salt former." In the gaseous state, the halogens form very reactive diatomic covalent molecules and can be identified by their distinctive colors. Halogens are found in Group 17.
Fluorine is the most chemically active of all the elements. Hydrofluoric acid, a mixture of hydrogen fluoride and water, is used to etch glass and to frost the inner surfaces of light-bulbs. Other fluorides are added to toothpastes and to city water systems to prevent tooth decay.
Research It!
Does your community add fluorides to its water?
The Noble Gases
Why are the noble gases called noble? It was known that these gases did not naturally form compounds. Thus, they were thought of as the nobility of elements because nobles did not mix with common folk. However, in the early 1960s, scientists were able to prepare some compounds of noble gases. As you recall, each element in Group 18 is stable because its outer energy level is full. The stability of the noble gases plays an important role in their uses.
Both the halogens and the noble gases illustrate that each element in a group has some similar properties but also has unique properties and uses.
Can an element be both a metal and a nonmetal? In a sense, some elements are. They are the metalloids, and they have both metallic and nonmetallic properties. A metalloid may conduct electricity better than many nonmetals but not as well as some metals. In the periodic table, the metalloids are the elements located along the stair-step line. The mixed groups: 13, 14, 15, and 16 - contain metals, nonmetals, and metalloids.
The Boron Group
Boron, a metalloid, is the first element in Group 13. If you look around your home, you may find two compounds of boron. One of these is boric acid, a mild antiseptic. The other is borax, which is used in laundry products to soften water. Less familiar are the boranes, which are compounds of boron used in fuels for rockets and jet airplanes.
The Carbon Group
Each element in Group 14, the carbon family, has four electrons in its outer energy level. But this is where much of the similarity ends. Carbon is a nonmetal; silicon and germanium are metalloids; tin and lead are metals.
The Nitrogen Group
The nitrogen family makes up Group 15. Each element has five electrons in its outer energy level. These elements tend to share electrons and to form covalent compounds with other elements.
The Oxygen Group
The oxygen group makes up Group 16 on the periodic table. You can live for only a short time without the nonmetal oxygen, which makes up about 20 percent of air. Oxygen exists in the air as diatomic molecules, O₂. During electrical storms, some oxygen molecules, O₂, change into ozone molecules, O₃. Do you notice that O₂ and O₃ are allotropes?
Synthetic Elements
What if you made something that always fell apart? You might think you were not successful. Yet nuclear scientists are learning to do just that. By combining existing elements with fast-moving particles, they have been successful at creating elements not typically found on Earth. Except for one, the short-lived elements have more than 92 protons. All of these synthetic elements are unstable and fall apart quickly. Elements having more than 92 protons, the atomic number of uranium, are called transuranium elements.
If you look your periodic table, you will see breaks in periods 6 and 7. The first break includes a series of 14 elements with atomic numbers of 58-71. The elements in this series are known as the lanthanides. The second break includes elements with atomic numbers ranging from 90-103. These elements are known as actinides. Ten synthetic elements are found in the actinide series. Where are the others placed on the periodic table? Technetium is element number 43. The newest synthetic elements are placed in period 7.
By studying how the synthesized elements form and fall apart, we can gain an understanding of the forces holding other elements together. When these atoms fall apart, they are said to be radioactive. Technetium's radioactivity makes it ideal for many medical applications. At this time, many of the synthetic elements, once made, last only small fractions of seconds and can be made only in small amounts. However, further advances in technology may bring production up to larger amounts. The as-yet-undiscovered applications could prove to be more valuable than their costs.