
Grade 11 | Lesson 6
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com

Grade 11 | Lesson 6
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com
Science
Lesson Overview
Electron Motion and Energy
• Satellites and Electrons
• Electrons and Light
• The Electron Cloud Model
![]()
Electron Motion and Energy
Considering that electrons are negative and that an atom’s nucleus contains positively charged protons, why aren’t electrons pulled into the nucleus and held there? Scientists in the early 20th century wondered the same thing.
Niels Bohr (1885 – 1962), a Danish scientist who worked with Rutherford, proposed that electrons must have enough energy to keep them in constant motion around the nucleus. He compared the motion of electrons to the motion of planets orbiting the sun. Although the planets are attracted to the sun by gravitational force, they move with enough energy to remain in stable orbits around the sun. In the same way, when we launch satellites, we use rockets to give them enough energy of motion so that the satellites stay in orbit around Earth. In a similar way, electrons have energy of motion that enables them to overcome the attraction of the positive nucleus. This energy keeps the electrons moving around the nucleus. Bohr’s view of the atom, which he proposed in 1913, was called the planetary model.
When a satellite is launched into orbit, the amount of energy determines how high above Earth it will orbit. Given a little more energy, the satellite will go into a slightly higher orbit’ with a little less energy, it will have a lower orbit . However, it seemed that electrons did not behave the same way. Instead, experiments showed that electrons occupied orbits of only certain amounts of energy. Bohr’s model had to explain these observations.
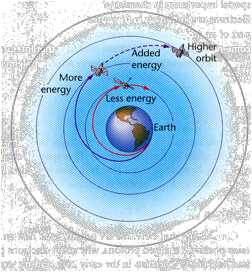
Satellites and Electrons
A satellite can orbit Earth at almost any altitude, depending on the amount of energy used to launch it. Electrons, on the other hand, can occupy orbits of only certain energies.
To boost a satellite into a higher orbit requires energy from a rocket motor. One way to increase the energy of an electron is to supply energy in the form of high-voltage electricity. Another way is to supply electromagnetic radiation, also called radiant energy. Radiant energy travels in the form of waves that have both electrical and magnetic properties. These electromagnetic waves can travel through empty space, as you know from the fact that radiant energy from the sun travels to Earth every day. As you may already have guessed, electromagnetic waves travel through space at the speed of light, which is approximately 300 million meters per second.
If you’ve ever seen water waves breaking on a shoreline or heard objects in a room vibrate from the effects of loud sound waves, you already know that waves transfer energy from one place to another. Electromagnetic waves have the same characteristics as other waves. Electromagnetic radiation includes radio waves that carry broadcasts to your radio and TV, microwave radiation used to heat food in microwave oven, radiant heat used to toast bread, and the most familiar form, visible light. All of these forms of radiant energy are parts of a whole range of electromagnetic radiation called the electromagnetic spectrum.
Only a small part of the electromagnetic spectrum is made up of visible light. Note that higher-frequency electromagnetic waves have higher energy than lower-frequency waves. This is an important fact to remember when you study the relationship of light to atomic structure.
Research It!
Who, along with Borh was pivotal in discovering electromagnetic radiation and the electromagnetic spectrum?
Electrons and Light
What does the electromagnetic spectrum have to do with electrons? It’s all related to energy – the energy of motion of the electron and the energy of light. Scientists passed a high-voltage electric current through hydrogen, which absorbed some of that energy. These excited hydrogen atoms returned the absorbed energy in the form of light. Passing that light through a prism revealed that the light consisted of just a few specific frequencies – not a whole range of colors as with white light. The spectrum of light released from excited atoms of an element is called the emission spectrum of that element.
What could explain the fact that the emission spectrum of hydrogen consists of just a few lines? Bohr theorized that electrons absorbed energy and moved to higher energy states. Then, these excited electrons gave off that energy as light waves when they fell back to a lower energy state. But, why were only certain frequencies of light given off? To answer this question, Bohr suggested that electrons could have only certain amounts of energy. When they absorb energy, electrons absorb only the amount needed to move to a specific higher energy state. Then, what the electrons fall back to a lower energy state, they emit only certain amounts of energy, resulting in only specific colors of light.
Because electrons can have only certain amounts of energy, Bohr reasoned, they can move around the nucleus only at distances that correspond to those amounts of energy. These regions of space in which electrons can move about the nucleus of an atom are called energy levels. Energy levels in an atom are like rungs on a ladder. You can compare the movement of electrons between energy levels to climbing up and down that ladder.
The Electron Cloud Model
As a result of continuing research throughout the 20th century, scientists today realize that energy levels are not neat planet like orbits around the nucleus of an atom. Instead, they are spherical regions of space around the nucleus in which electrons are most likely to be found. Electrons themselves take up little space but travel rapidly through the space surrounding the nucleus. These spherical regions were electrons travel may be depicted as clouds around the nucleus. The space around the nucleus of an atom where the atom’s electrons are found is called the electron cloud.
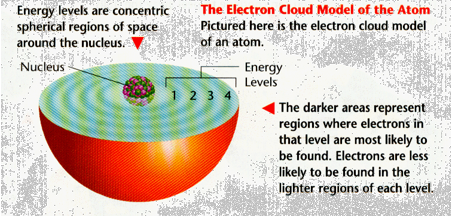
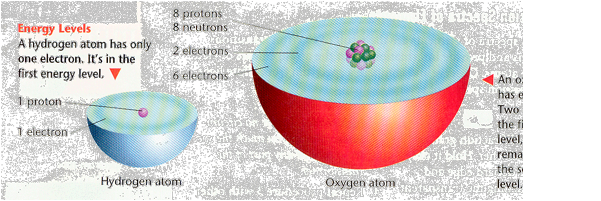
How are electrons arranged in energy levels? Each energy level can hold a limited number of electrons. The lowest energy level is the smallest and the closest to the nucleus. This first energy level holds a maximum of two electrons. The second energy level is larger because it is farther away from the nucleus. It holds a maximum of eight electrons. The third energy level is larger still and holds a maximum of 18 electrons.
For now, it’s important to learn about the electrons in the outermost energy level of an atom. The electrons in the outermost energy level are called valence electrons. Hydrogen has one valence electron and oxygen has six valence electrons. You can also use the periodic table as a tool to predict the number of valence electrons in any atom in Groups 1, 2, 13, 14, 15, 16, 17, and 18. All atoms in Group 1, like hydrogen, have one valence electron. Likewise, atoms in Group 2 have two valence electrons. Atoms in Groups 13-18 have three through eight valence electrons, respectively.
Why do you need to know how to determine the number of outer-level electrons that are in an atom? Remember that when atoms come near each other, it is the electrons that interact. In fact, it is the valence electrons that interact. Therefore, many of the chemical and physical properties of an element are directly related to the number and arrangement of valence electrons.
Because valence electrons are so important to the behavior of an atom, it is useful to represent them with symbols. A Lewis dot diagram illustrates valence electrons as dots (or other small symbols) around the chemical symbol of an element. Each dot represents one valence electron. In the dot diagram, the element’s symbol represents the core of the atom – the nucleus plus all the inner electrons.
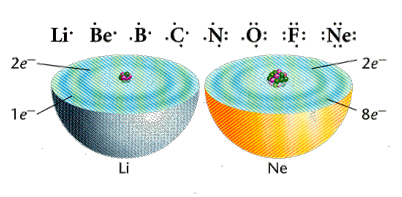
The Lewis dot diagrams for these elements illustrate how valence electrons change from element to element across a row on the periodic table.
A Lewis dot diagram is a convenient, shorthand method to represent an element and its valence electrons. You have used the periodic table already as a source of information about the symbols, names, atomic numbers, and average atomic masses of elements. You will learn that the arrangement of elements on the periodic table yields even more information about the electronic structures of atoms and how those structures can help you to predict many of the properties of elements.