
Grade 8 | Lesson 19
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com

Grade 8 | Lesson 19
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com
Science
Lesson Overview
Solutions
• Dissolving
• Concentration
• The Nature of Solvents and Solutes
![]()
Solutions
When you hear the word solution, you may think of a liquid mixture, such as salt water or soda. Both of these liquids are examples of solutions. But did you know that the air you breathe is also a solution? So is the sterling silver used to make fine jewelry. Solutions, then, can be gaseous or solid as well as liquid. A solution is a homogeneous mixture, in which the particles of the mixing substances are evenly distributed throughout.

To describe a solution, you may say that one substance is dissolved in another. The substance being dissolved is the solute. The substance that dissolves the solute is the solvent. When a solid dissolves in a liquid, the solid is the solute and the liquid is the solvent. Thus, in salt water, sodium chloride is the solute, and water is the solvent. In a liquid-gas solution, the gas is the solute. In soda, carbon dioxide is the solute and water is the solvent.
Dissolving
The dissolving of a solid in a liquid occurs at the surface of the solid. For water solutions, keep in mind two things you have learned about water. Like the particles of any liquid, water molecules are constantly moving. A water molecule is polar, which means it has a positive area and a negative area. Molecules of sugar are also polar.
The process repeats itself as layer after layer of sugar molecules moves away from the crystal, until all the molecules are evenly spread out. The same three steps occur when any polar or ionic compound dissolves in a polar liquid. A similar, but more difficult, process also occurs when a gas dissolves in a liquid.
When you add the sugar to the water, you stir it. Stirring a solution speeds up dissolving because it brings more fresh solvent into contact with more solute. The fresh solvent attracts the particles in the solute, causing the solid solute to move into solution faster. A second way to speed the dissolving of a solid in a liquid is to grind large crystals into smaller ones.
Breaking the crystal into smaller pieces greatly increases its surface area. Because dissolving takes place at the surface of the solid, increasing the surface area allows more solvent to come into contact with more solid solute. Thus, the speed of the solution process increases.
A third way to increase the rate at which most solids dissolve is to increase the temperature of a solvent. You can make the sugar dissolve faster by putting in hot water instead of cold water. Increasing the temperature of a solvent speeds up the movement of its particles. This increase causes more solvent particles to bump into the solute. As a result, solute particles break loose faster.
What might you do if you wanted the gas to dissolve faster in the liquid? The answer is simple: cool the liquid solvent and increase the pressure of the gas. In a soda-bottling plant, both of these things are done. The machinery cools the solution and keeps it under pressure. An unopened bottle of soda has no bubbles. In the sealed bottle, pressure keeps all of the gas dissolved. When the bottle is opened, the pressure of the carbon dioxide is greatly reduced. Then the carbon dioxide comes out of solution quickly. Generally, the solubility of a substance is expressed as the maximum number of grams of the substance that will dissolve in 100 g of solvent at a certain temperature.
Concentration
A concentrated solution is one in which there is a large amount of solute in the solvent. A dilute solution is one in which there is a small amount of solute in the solvent. Concentrated and dilute are not precise terms. However, solution concentrations can be described precisely. One way is to state the percentage by volume of the solute.
You can express concentration of a solid dissolved in a liquid as percentage by mass. This percentage is the mass in grams of solute plus enough solvent to make 100 g of solution. Because 1 mL of water has a mass of 1 g, you would mix 10 g of sodium chloride with 90 mL of water to make a ten percent solution of sodium chloride.
How much solute can dissolve in a given amount of solvent? That depends on a number of factors. Let's examine the types of solutions based on the amount of solute dissolved.
If you add 30.0 g of potassium chloride, KCl, to 100 g of water at 0°C, only 28 g will dissolve. You have a saturated solution because no more KCl can dissolve. A saturated solution is a solution that has dissolved all the solute it normally can hold at a given temperature. But if you heat the mixture to a higher temperature, more KCl can dissolve. As the temperature of the liquid solvent increases, the amount of solute that can dissolve in it usually increases. The table below gives the amounts of a few solutes that can dissolve in water at different temperatures, forming saturated solutions.
Solubility in g/100 g of Water at the Temperature Indicated |
|||||
Compound |
Formula |
0°C |
20°C |
60°C |
100°C |
Ammonium chloride |
NH₄Cl |
29.4 |
37.2 |
55.3 |
77.3 |
Barium hydroxide |
Ba(OH)₂ |
1.67 |
3.89 |
20.94 |
101.4 |
Copper(II) sulfate |
CuSO₄ |
23.1 |
32.0 |
61.8 |
114 |
Lead(II) chloride |
PbCl₂ |
0.67 |
1.00 |
1.94 |
3.20 |
Potassium bromide |
KBr |
53.6 |
65.3 |
85.5 |
104 |
Potassium chloride |
KCl |
28.0 |
34.0 |
45.8 |
56.3 |
Potassium nitrate |
KNO3 |
13.9 |
31.6 |
109 |
245 |
Sodium acetate |
NaC₂H₃O₂ |
36.2 |
46.4 |
139 |
170.15 |
Sodium chlorate |
NaClO₃ |
79.6 |
95.9 |
137 |
204 |
Sodium chloride |
NaCl |
35.7 |
35.9 |
37.1 |
39.2 |
Sodium nitrate |
NaNO₃ |
73.0 |
87.6 |
122 |
180 |
Sucrose (sugar) |
C₁₂H₂₂O₁₁ |
179.2 |
203.9 |
287.3 |
487.2 |
An unsaturated solution is any solution that can dissolve more solute at a given temperature. Each time a saturated solution is heated to a higher temperature, it may become unsaturated. The term unsaturated isn't precise. If you look at the table above you'll see that at 20°C, 37.2 g of NH₄Cl dissolved in 100 g of water is a saturated solution. However, an unsaturated solution of NH₄Cl could be any amount less than 37.2 g in 100 g of water at 20°C.
If you make a standard solution of potassium nitrate at 100°C and then let it cool to 20°C, part of the solute comes out of solution. At the lower temperature, a saturated solution is formed with less solute. Most other saturated solutions behave in a similar way when cooled. But if you cool a saturated solution of sodium acetate form 100°C to 20°C, with proper precautions, no solute comes out. This solution is supersaturated. A supersaturated solution contains more solute than a saturated one has at that temperature. This kind of solution is unstable. When a small crystal of the solute is added to a supersaturated solution, the excess solute quickly crystallizes out.
How can you determine whether a solution is saturated, unsaturated, or supersaturated? Suppose you add a solute crystal to a solution. If the crystal dissolves, the solution is unsaturated. If the crystal does not dissolve, the solution is saturated. And if excess solute comes out, the solution is supersaturated.
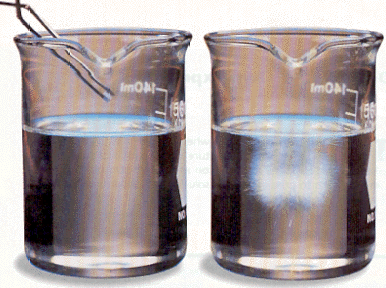
A solution of sodium acetate (left) crystallizes out when a crystal of sodium acetate is added (right).
The Nature of Solvents and Solutes
Why does a substance dissolve in one solvent but not in another? Solvents with nonpolar molecules dissolve solutes with nonpolar molecules. A solvent such as hexane dissolves grease because both the solvent and the solute have nonpolar molecules. Similarly, polar solvents dissolve polar solutes. Water is a polar solvent, and it dissolves sucrose, a polar solute. Polar solvents also dissolve ionic solutes, such as sodium chloride. A polar solvent won't normally dissolve a nonpolar solute. That's why oil, which is nonpolar, won't dissolve in vinegar, which is polar. In general, when predicting which solutes will dissolve in which solvents, remember the phrase, "like dissolves like."
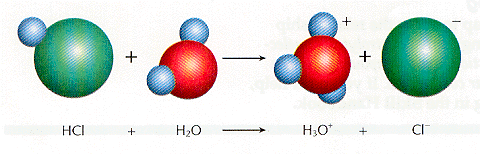
As an ionic solid dissolves in water, the positive and negative ions separate from one another. This separation process is called dissociation. For example, sodium chloride dissociates when it dissolves in water. When certain polar substances dissolve in water, the water pulls their molecules apart. This process is called ionization. Hydrogen chloride ionizes in water, as shown below. a substance that separates into ions or forms ions in a water solution is called an electrolyte. Thus, both NaCl and HCl are examples of electrolytes.
Pure water is a nonelectrolyte, which means it does not conduct electricity. If a water solution is a conductor, the solute must be an electrolyte.
Antifreeze that you may add to water in a car radiator makes freezing more difficult. Adding a solute to a solvent lowers the freezing point of the solvent. How much the freezing point goes down depends upon how many solute particles you add. How does antifreeze work? As a substance freezes, its particles organize themselves into an orderly pattern. The solute particles interfere with the formation of this orderly pattern. This prevents the solvent from freezing at its normal freezing point. To overcome this interference, a lower temperature is required to freeze the solvent. The same principle applies to certain animals that live in extremely cold climates. Caribou, for example, contain substances in the lower section of their legs that prevent their freezing in subzero temperatures. The caribou can stand for long periods of time in snow and ice with no harm to their legs.
You may also know that antifreeze raises the boiling point of the water in a car radiator. What causes this effect? The amount that the boiling point is raised depends upon the number of solute molecules present. Solute particles interfere with the evaporation of solvent particles. Thus, more energy is needed to allow the solvent particles to evaporate, and the solution boils at a higher temperature.